Calcium Sulfide Lewis Dot Structure
what is the Lewis dot structure for one formula unit of calcium sulfide?
Related Question
what is the lewis dot construction of strontium sulfide
Discussion
You must exist signed in to hash out.
Video Transcript
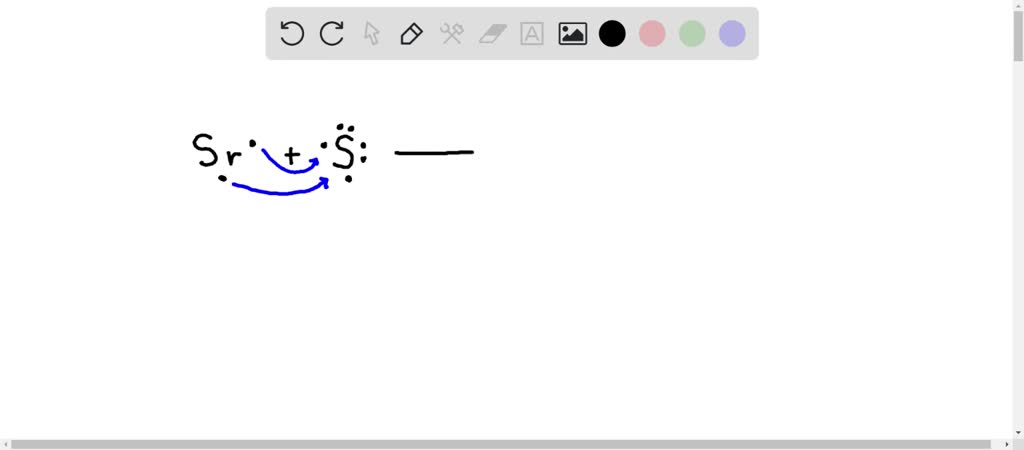
Howdy there. In this question. Nosotros want to write the dot structure for strontium sulfide. This is an ionic compound considering it'due south equanimous of a metal and a nonmetal. So, I desire to testify the production of strontium sulfide. Using the dot structures strontium Is in Group two a. of the periodic tabular array. So information technology has two valence electrons, sulfur Is in Grouping half-dozen a. of the periodic table. So it has six valence electrons. Right? So this would be the dot structure of each of these when they form a compound, strong Tm is going to give information technology to electrons to sulfur. So what that is going to give us his strontium With a two plus charge and only its core electrons remaining. So no dots around it. And sulfur With eight dots around it. Gonna put brackets around it to show that all day all the dots belong to the sulfur And it has a two negative charge. Alright, so this would exist our final lewis dot structure for strontium sulfide. Once again, since it's ionic, they are not sharing electrons. Electrons were completely transferred from the strontium to the sulfide. All correct, thanks so much for asking your question and I hope this was helpful
Calcium Sulfide Lewis Dot Structure,
Source: https://www.numerade.com/ask/question/what-is-the-lewis-dot-structure-for-one-formula-unit-of-calcium-sulfide-70802/
Posted by: youngsibrom.blogspot.com



0 Response to "Calcium Sulfide Lewis Dot Structure"
Post a Comment